Cell Metabolism New insight into endothelial cell metabolism in angiogenesis
Resource: State Key Laboratary of Ophthalmology
Written by: State Key Laboratary of Ophthalmology
Proofread by: Jiawei Wang
Edited by: Xianjing Wei
On September 3, 2019, an reivew article titled “Hallmarks of Endothelial Cell Metabolism in Health and Disease” was published by the journal of Cell Metabolism. In this article, Dr. Xuri Li at the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center (ZOC), Sun Yat-Sen University (SYSU) and Dr. Peter Carmeliet at VIB-KU Leuven in Belgium reveal the critical roles of vascular endotelial metabolism in angiogenesis and therapeutic implications in the treatment of neovascular diseases.

In 2009, it was postulated that vascular endothelial cells (ECs) would only be able to execute the orders of growth factors if these cells would accordingly adapt their metabolism. Ten years later, it has become clear that ECs, often differently from other cell types, rely on distinct metabolic pathways to survive and form new blood vessels. Manipulation of EC metabolic pathways alone (even without changing angiogenic signaling) suffices to alter vessel sprouting. Perturbations of these metabolic pathways can underlie excess formation of new blood vessels (angiogenesis) in cancer and ocular diseases. Initial proof of evidence has been provided that targeting (normalizing) these metabolic perturbations in diseased ECs and delivery of metabolites deserve increasing attention as novel therapeutic approaches for inhibiting or stimulating vessel growth in multiple disorders.
Traditionally, growth factors, membrane receptors, and downstream signaling cascades have been at the center stage of the (lymph-)angiogenesis field. Because of their importance, they also have become drug targets to inhibit excessive angiogenesis in cancer and blinding eye diseases. This is best illustrated by the discovery of VEGF as one of the key angiogenic growth factors and the identification of its essential role in angiogenesis in health and disease. This constituted the basis for the development of VEGF-targeted anti-angiogenic drugs during the last 30 years. However, despite the success of various clinically approved anti-angiogenic drugs targeting growth factors or their receptors, they often lack sufficient efficacy, suffer resistance, and induce some degree of systemic toxicity in both cancer and eye disease patients. While multiple explanations for these shortcomings have been proposed, an important mechanism is that blockade of one growth factor (receptor) may compensatorily upregulate the expression of others, which may stimulate angiogenesis again ( A and B in the below figure).
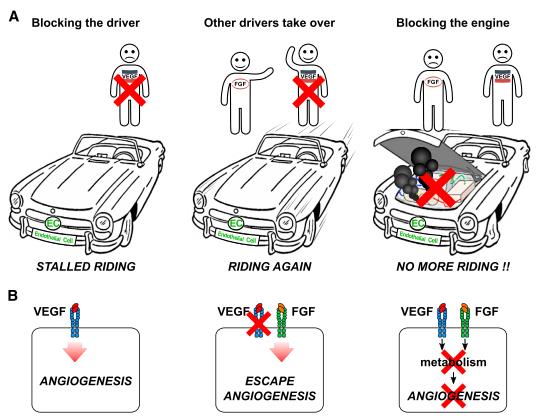
Therefore, the growth factor-centric anti-angiogenic approach was critically reconsidered, and the need for fundamentally innovative approaches was realized, no longer by targeting the drivers (growth factors, their receptors, anddownstream signaling), but, instead, by targeting the engine (EC metabolism), which is vital for ECs to form new vessels. Or, rephrased using a simile (A and B in te above figue), a sprouting blood vessel is like a car that requires an engine and a driver to go. In this model, blocking VEGF to impair vessel sprouting is like removing a driver to arrest the car. However, as long as other angiogenic signals (car drivers) are available, blood vessels continue to grow (the car will still drive). Instead, if the engine of the car is jammed, the car will no longer be able to drive, regardless of how many drivers are still available. Or, rephrased for anti-angiogenesis therapy, if EC metabolism is targeted, the blood vessel will no longer be able to grow, regardless of how many angiogenic signals are still present. In summary, EC metabolism is crucial for angiogenesis and targeting EC metabolic pathways may have promising therapeutic utilities in treating neovascular diseases.
Full article: https://www.cell.com/cell-metabolism/fulltext/S1550-4131(19)30440-1
