Molecular Therapy | Professor Zhuo Yehong's team from Zhongshan Ophthalmic Center proposed a new pathogenesis and new treatment method for POAG based on DNA methylation
Resource: State Key Laboratory of Ophthalmology
Written by: State Key Laboratory of Ophthalmology
Proofread by: Jiawei Wang
Edited by: Xianjing Wei
Glaucoma is the main cause of permanent visual impairment, affecting more than 70 million people worldwide [1]. Aqueous humor outflow disorder is considered to be the main cause of primary open angle glaucoma (primary open angle glaucoma, POAG). If left untreated, increased intraocular pressure will cause the gradual loss of retinal ganglion cells and axons [2 ], Resulting in irreversible visual damage.
On January 1, 2021, Prof. Zhuo Yehong’s team from the Zhongshan Ophthalmology Center of Sun Yat-Sen University published a research article in Molecular Therapy, titled "TET-dependent GDF7 hypomethylation impairs aqueous outflow and serves as a potential therapeutic target in glaucoma" research paper.
This work describes the DNA methylation profile of trabecular tissues in patients with POAG for the first time, and identifies that abnormal growth differentiation factor 7 (GDF7) gene methylation is an important link in the pathogenesis of POAG. And the development of GDF7 antibody neutralizing treatment has achieved therapeutic effects in the rhesus monkey model.

In this study, the researchers performed genomewide DNA methylation analysis on the trabecular meshwork tissue from POAG patients and found 810 abnormal methylation sites, most of which are related to trabecular meshwork fibrosis. Among them, the hypomethylation of GDF7 was selected as a key factor in the onset of humor outflow disorders. The methylation level of GDF7 gene is closely related to the clinical manifestations of patients (such as intraocular pressure, retinal nerve fiber layer thickness, cup-to-disk ratio, and degree of visual field defect) (Figure 1).
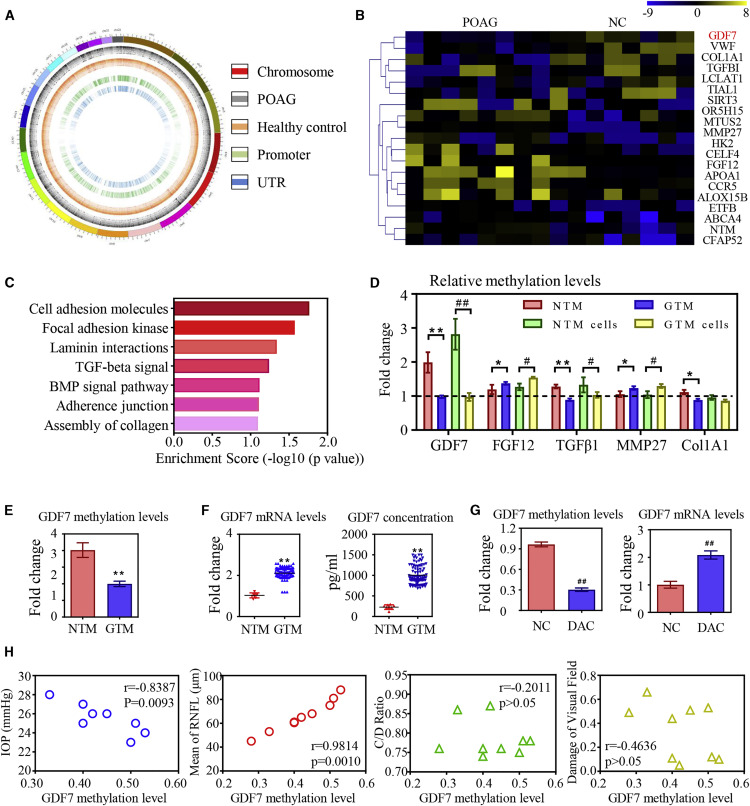
Figure 1
Further functional studies have confirmed that the decrease in GDF7 gene methylation level leads to excessive synthesis and secretion of GDF7 protein, which resulted in TM fibrosis and obstruction of aqueous humor outflow (Figure 2).
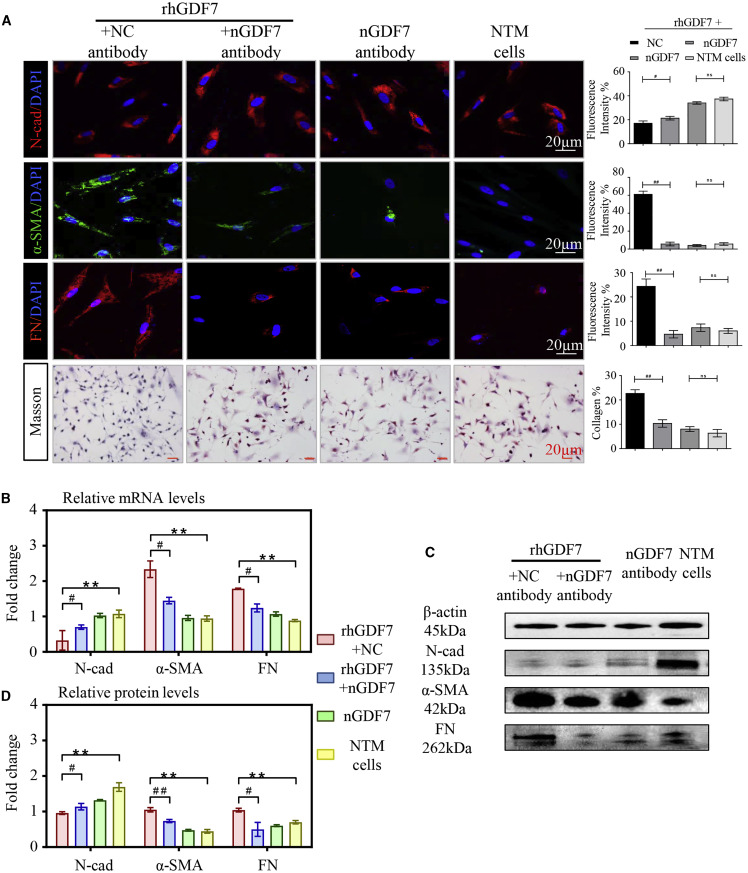
Figure 2
This methylation-dependent mechanism has also been confirmed in silico through a machine learning model, with a specificity of 84.38% and a sensitivity of 89.38% (Figure 3).
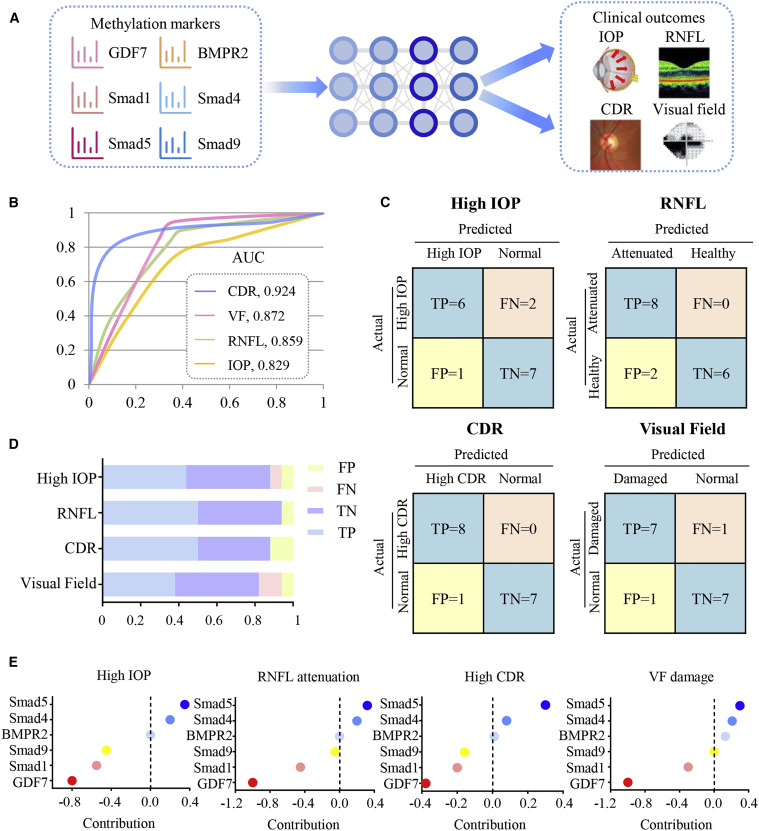
Figure 3
The researchers also developed GDF7 antibody neutralization therapy to inhibit the trabecular meshwork fibrosis and improve the aqueous humor outflow. In the rhesus monkey model, GDF7 neutralization therapy achieved effective control of intraocular pressure (from 21.3±0.3 to 17.6±0.2 mmHg), aqueous humor outflow facility was increased by three times (from 0.1 to 0.3μL/min•mmHg), and effective protection of optic nerve fibers (Figure 4).
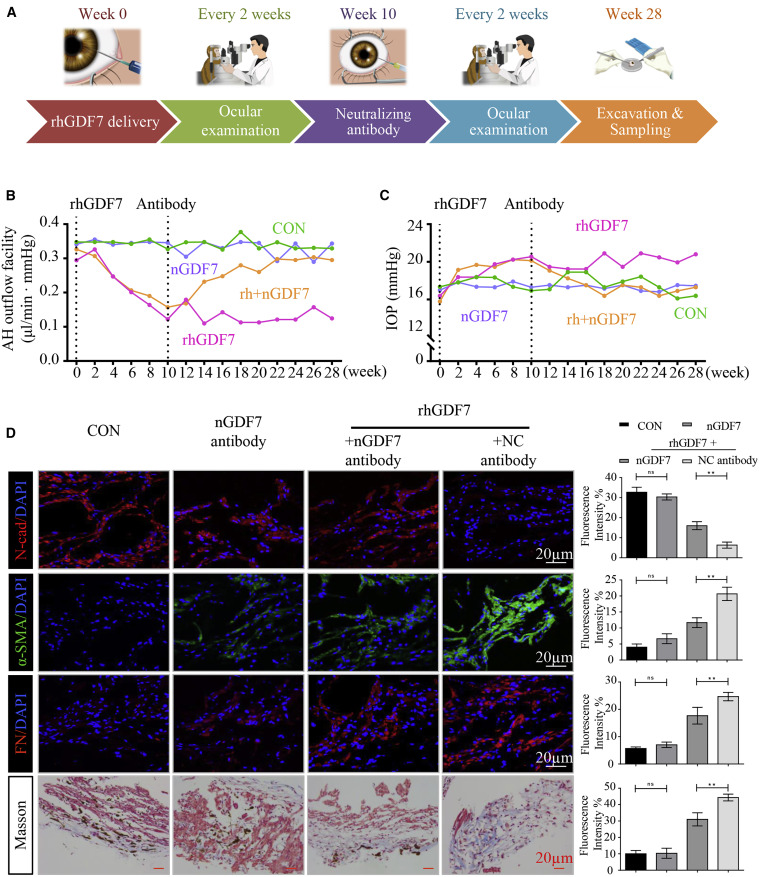
Figure 4
For the first time, this research provides new insights into the pathogenesis of glaucoma from the perspective of abnormal DNA methylation, and proposes an innovative GDF7 antibody neutralization therapy as an intervention with clinical application prospects.
Paper Link:
https://www.sciencedirect.com/science/article/pii/S1525001620306882?via%3Dihub
References:
[1] Y.H. Kwon, J.H. Fingert, M.H. Kuehn, W.L. Alward. Primary open-angle glaucoma. N. Engl. J. Med., 360 (2009), pp. 1113-1124.
[2] B.M. Braunger, R. Fuchshofer, E.R. Tamm. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm., 95 (Pt B) (2015), pp. 173-181.
[3] G. Gong, S. Kosoko-Lasaki, G. Haynatzki, H.T. Lynch, J.A. Lynch, M.R. Wilson. Inherited, familial and sporadic primary open-angle glaucoma. J. Natl. Med. Assoc., 99 (2007), pp. 559-563.
[4] A.P. Khawaja, J.N. Cooke Bailey, N.J. Wareham, R.A. Scott, M. Simcoe, R.P. Igo Jr., Y.E. Song, R. Wojciechowski, C.Y. Cheng, P.T. Khaw, et al., UK Biobank Eye and Vision Consortium, NEIGHBORHOOD Consortium. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet., 50 (2018), pp. 778-782.
