PNAS | Prof. Xialin Liu’s team proposed a novel biological process for causing retinopathy committed by a specific RIP3+ subpopulation of microglia.
Resource: State Key Laboratory of Ophthalmology
Written by: State Key Laboratory of Ophthalmology
Auditor: Xialin Liu
Proofread by: Jiawei Wang
Edited by: Xianjing Wei
Recently, Professor Xialin Liu’s team published a research article entitled " A specific RIP3 + subpopulation of microglia promotes retinopathy through a hypoxia-triggered necroptotic mechanism" on the famous international journal Proc Natl Acad Sci USA. This article revealed a new mechanism of a specific RIP3 + subgroup of microglia promoting retinal neovascularization in hypoxia-triggered retinopathy. Professor Xialin Liu and Professor Yizhi Liu from Zhongshan Ophthalmic Center, Sun Yat-sen University, and Professor Yihai Cao from Karolinska Institute, Sweden are the co-corresponding authors, and Dr. He Chang from Zhongshan Ophthalmic Center is the first author.
Retinal vascular diseases including retinopathy of prematurity (ROP), diabetic retinopathy (DR), and so on often lead to a catastrophic loss of vision. Targeting pathological angiogenesis offers an attractive approach for treating these retinopathies. Microglia are responsible for irregular sprouting and growth of the vasculature during pathological insults. However, the molecular mechanisms underlying microglia-mediated retinal neovascularization are largely unknown.
In this study, a special subgroup of RIP3 + microglia were identified for the first time in retinal vascular diseases, and its functional mechanism was elucidated. Under hypoxic conditions, RIP1/RIP3/MLKL pathway was activated in this subpopulation of microglia, which lead to pathological angiogenesis by releasing angiogenic factors such as FGF2. This study expands the understanding of microglia heterogeneity, enriches the atlas of microglia subpopulation, and updates the knowledge of cell death modes. It proposes that a supplement to anti-VEGF drugs by combining therapies targeting on microglial necroptosis is a new therapy for retinopathy.
The researchers used single cell RNA sequencing technology to classify microglia subsets in the oxygen induced retinopathy (OIR) mouse model and a specific subpopulation of microglia characterized by RIP3+ was identified. This result was further verified by immunofluorescence on retina flatmount, which showed Iba1+ RIP3 + microglia around the retinal angiogenic tufts. Then, in the OIR model of the microglial conditional RIP3 knockout mice, researchers found obviously reduced neovascularization, which confirmed this subgroup of cells mediated the process of pathological retinal angiogenesis (Figure. 1).
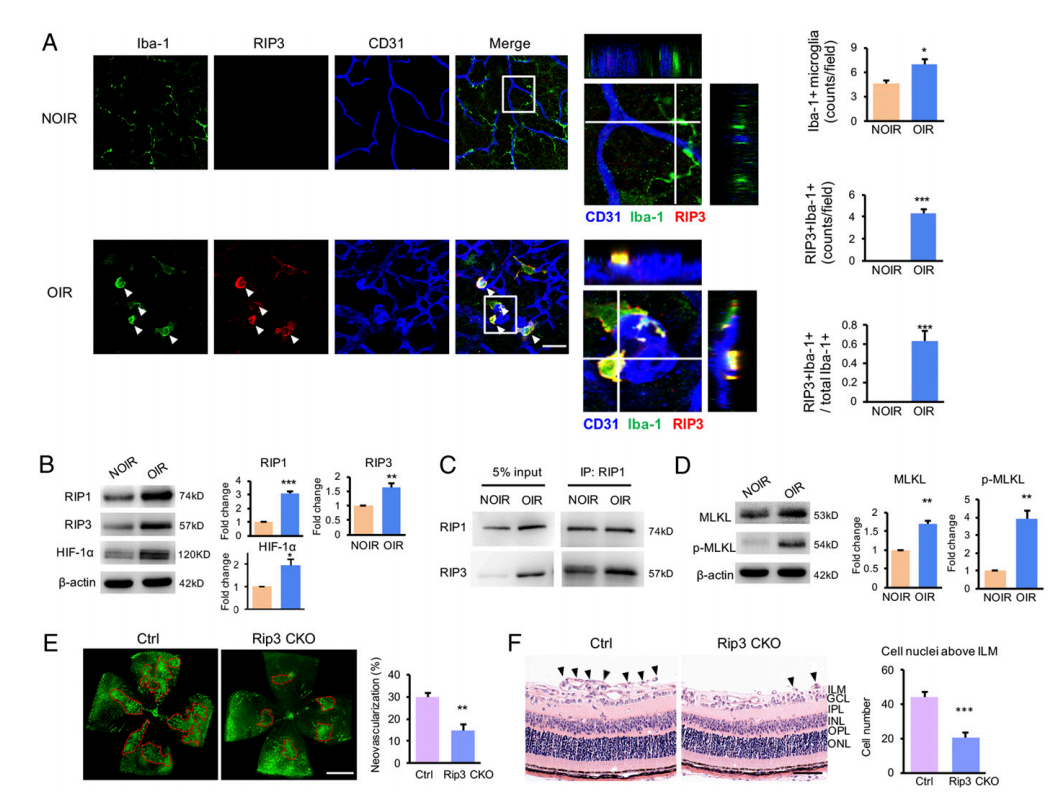
Figure 1. An activated subpopulation of microglia experienced necroptosis in response to hypoxia and promoted retinal neovascularization.
At the same time, the researchers isolated primary murine microglia cells and cultured microglia cell lines in vitro, and proved that hypoxia could induce the up-regulation of RIP1/RIP3/MLKL in microglia and lead to necroptosis. This process could be blocked by Nec-1, a RIP1 inhibitor (Figure. 2).
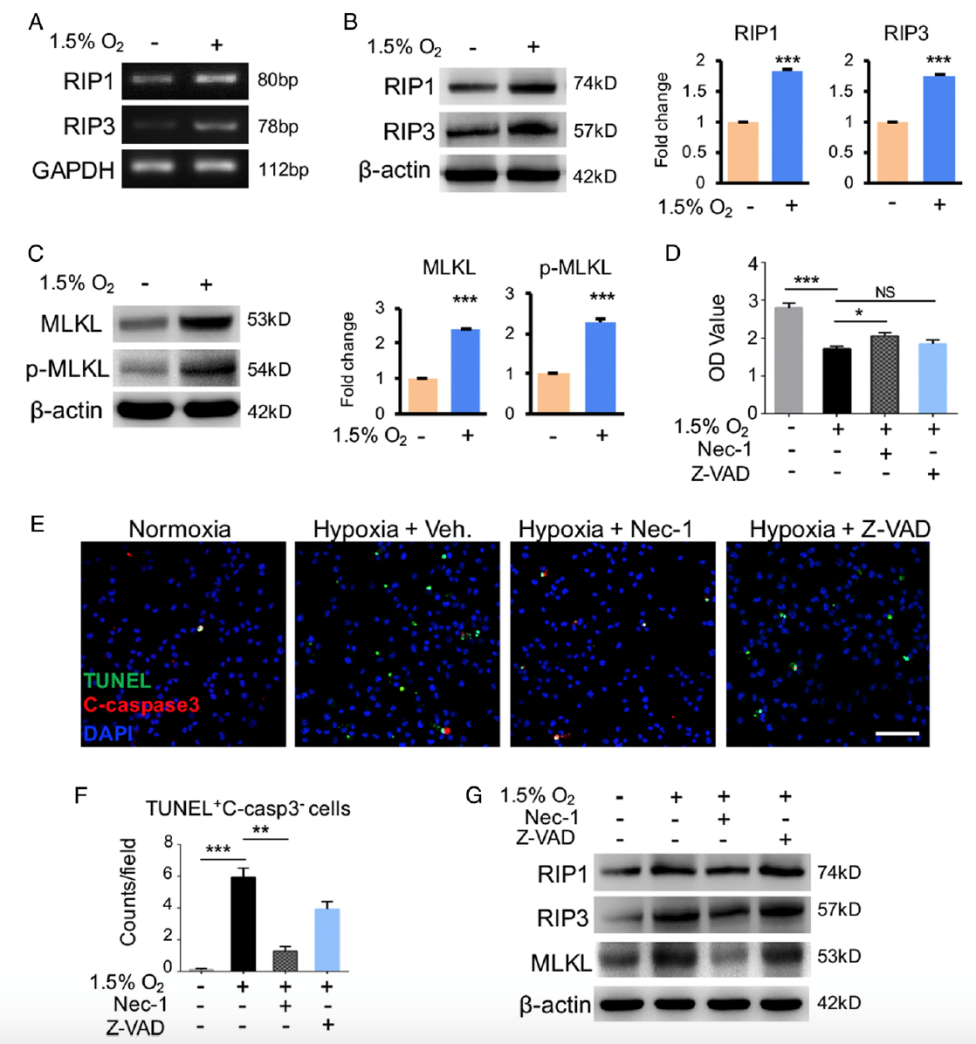
Figure 2. Hypoxia-induced necroptosis in cultured microglia.
To further explore how necroptotic RIP3+ microglia mediates retinal neovascularization, researchers used multiple cytokines assays to screen the expression of angiogenic factors, and found that FGF2 was most significantly up-regulated in OIR model. It was further confirmed that RIP3+ microglia highly expressed FGF2 in the retina of OIR mice, and the release of FGF2 expression was significantly reduced after Nec-1 application as well as in RIP3 CKO mice. These results suggested that RIP3+ microglia can release FGF2 through necroptosis (Figure. 3).
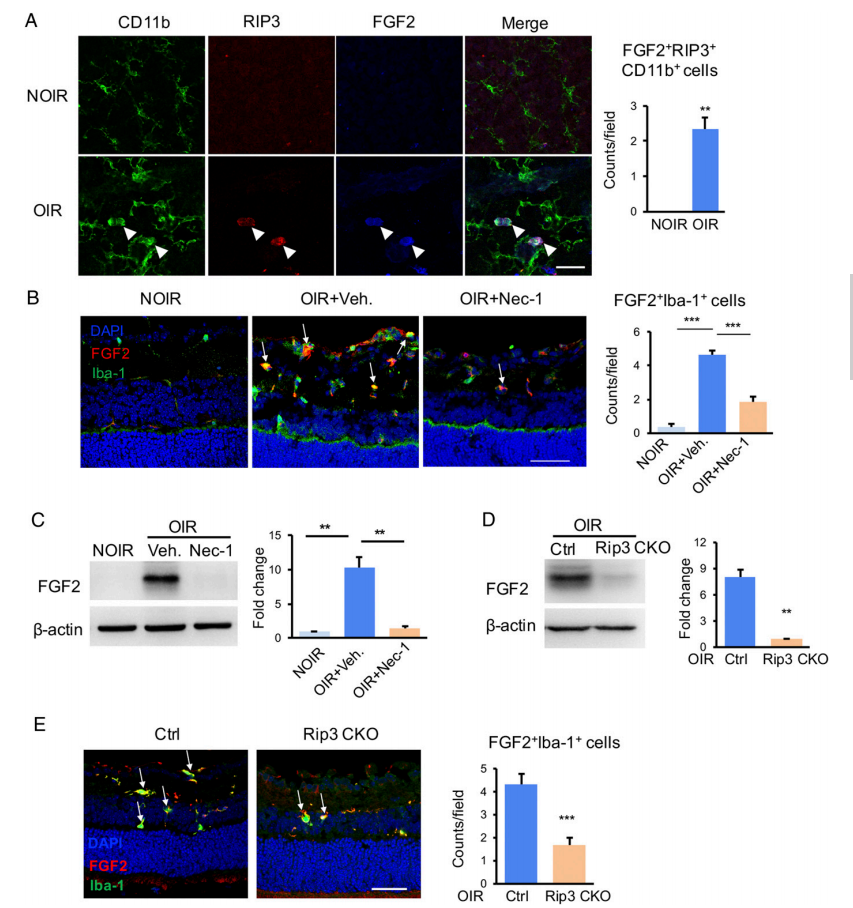
Figure 3. Improved FGF2 production in necroptotic microglia in vivo.
These results suggest that under hypoxic condition, necroptotic RIP3+ subpopulation of retinal microglia released FGF2 to promote retinal angiogenesis in a RIP1/RIP3/MLKL dependent manner. Based on these results, the researchers proposed a new strategy to treat retinal neovascularization by combining therapies targeting on microglia necroptosis and VEGF, which showed significant therapeutic effect in animal experiments (Figure. 4).
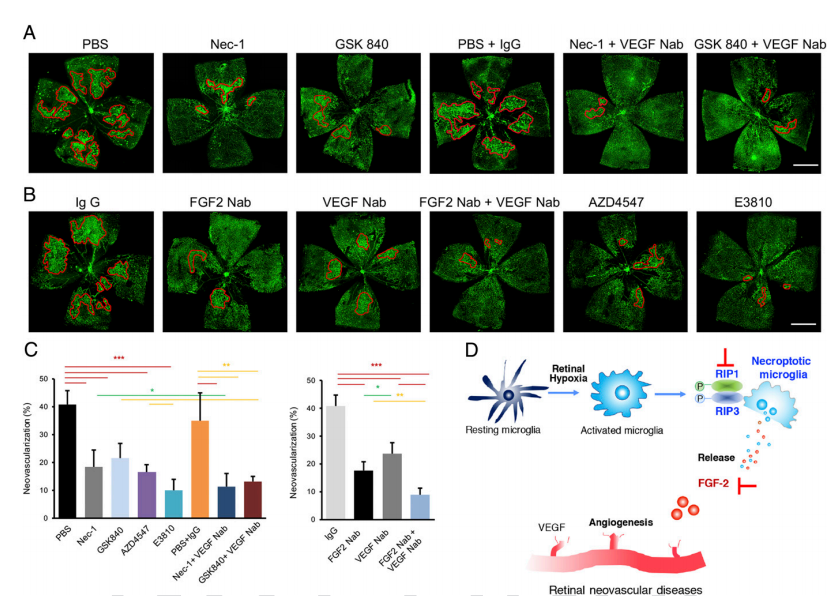
Figure 4. Combined inhibition of necroptosis–FGF2 signaling and VEGF signaling ameliorated pathologic angiogenesis in OIR.
In conclusion, this study firstly identified a novel microglial subset in retinal neovascularization. This RIP3+ microglia subset promoted retinal angiogenesis by releasing FGF2 via activating RIP1/RIP3/MLKL pathway.
This work was supported by National Key R&D Program of China; National Natural Science Foundation of China; Natural Science Foundation of Guangdong Province, China; and Science and Technology Program of Guangzhou, China.
Online Link: https://www.pnas.org/content/118/11/e2023290118
