Alleviating Experimental Allergic Eye Disease by Inhibiting Pro-lymphangiogenic VEGFR3 Signal
Resource: State Key Laboratory of Ophthalmology
Proofread by: Jiawei Wang
Edited by: Xianjing Wei
Reviewed by: Xiaoling Liang
Allergic eye disease (AED) is a common ocular disease that causes erythema, itching, eyelid edema, conjunctival chemosis and, in rare cases, corneal damage and impaired visual function. It affects up to 20% of the world’s population and significantly reduces the quality of life for patients. This disease comprises two phases of pathology development. Upon first exposure to an allergen, the ocular surface immune system becomes sensitized. This involves recognition of the antigen by neutrophils and macrophages, followed by activation of B cells and the synthesis of antigen-specific immunoglobulin E (IgE). With each new exposure, the allergen is captured by IgE-activated mast cells at conjunctiva mucosal surfaces, leading to mast cell degranulation and subsequent induction of Th2 type immune response. The conjunctiva has long been recognized as the most lymphatic-developed ocular tissue but how the conjunctival lymphatic system patriciates in the development of AED remains to be elucidated.
Recently, Prof. Feng Zhang’s team at the Zhongshan Ophthalmology Center of Sun Yat-Sen University published a research article entitled “Alleviating Experimental Allergic Eye Disease by Inhibiting Pro-lymphangiogenic VEGFR3 Signal” in Ocular Surface. This work reveals the key role of pro-lymphangiogenic VEGFR3 signaling in the development of AED and provides experimental evidence that VEGFR3 inhibition may be useful in treating ocular allergy in patients.

The researchers initiated the study by establishing optimized AED manifestations in mice via ovalbumin (OVA)+pertussis toxin (PTX) as a sensitizer and daily OVA challenges. They showed that conjunctival lymphatics underwent significant expansion after 28 days of chronic OVA challenge, characterized by significantly increased coverage and diameter of the conjunctival lymphatic vessels in the eyes. They also observed that OVA dosages (50 μg vs 250 μg) in the sensitizer mixture did not affect their efficiency in inducing lymphatic expansion in the allergic conjunctival tissue, demonstrating the dose-independent feature of the OVA effect (Figure 1).
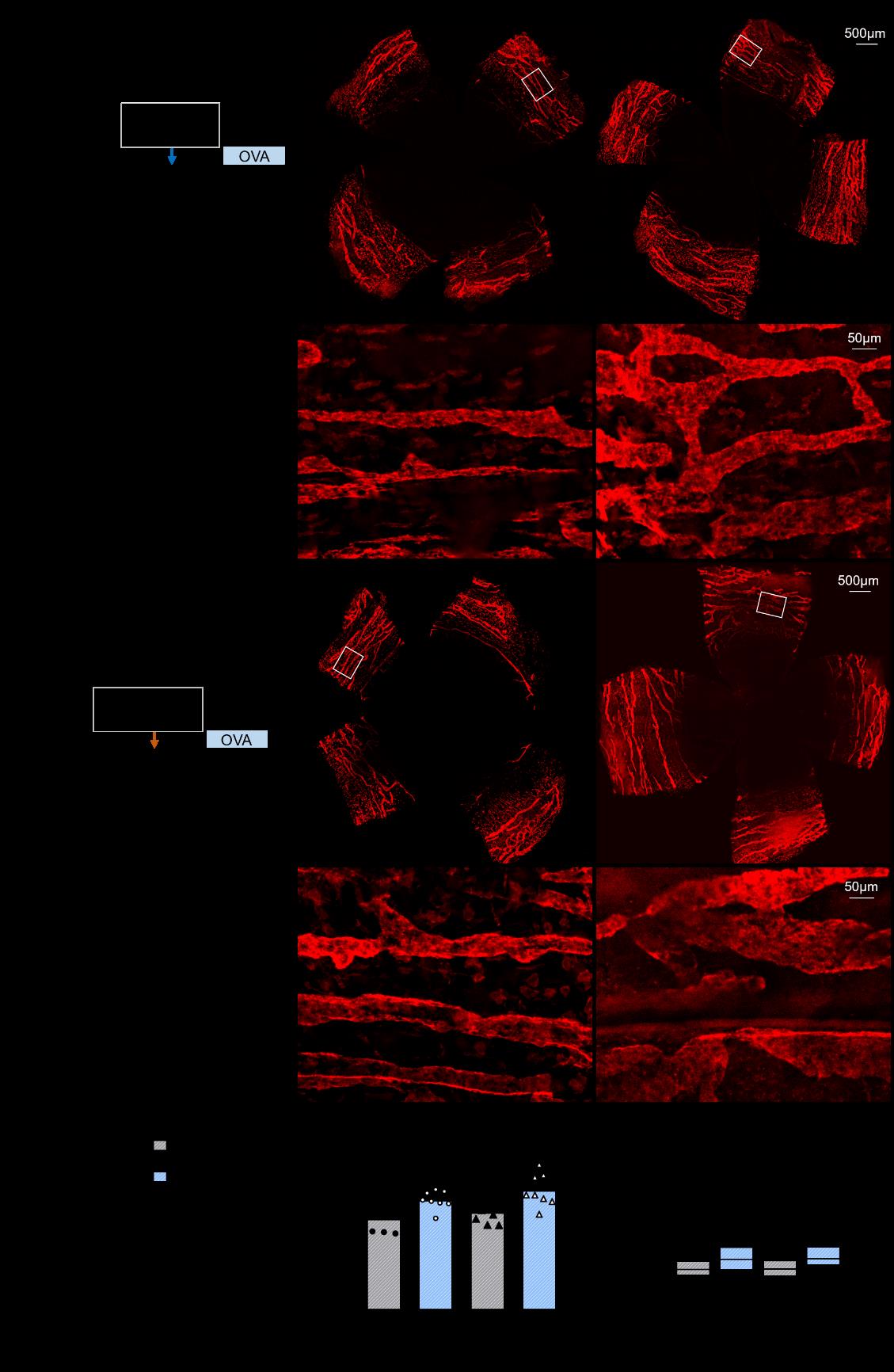
Figure 1 Ocular surface allergy induces expansion of conjunctival lymphatics
Next, they investigated whether VEGFR3, the major pro-lymphangiogenic factor, contributes to allergy-induced conjunctival lymphangiogenesis. They found deletion of Vegfr3 in lymphatic endothelial cells resulted in significantly decreased coverage and diameter of the conjunctival lymphatic vessels after OVA challenge, suggesting that allergy-induced expansion of conjunctival lymphatics is mediated by VEGFR3 signal (Figure 2).
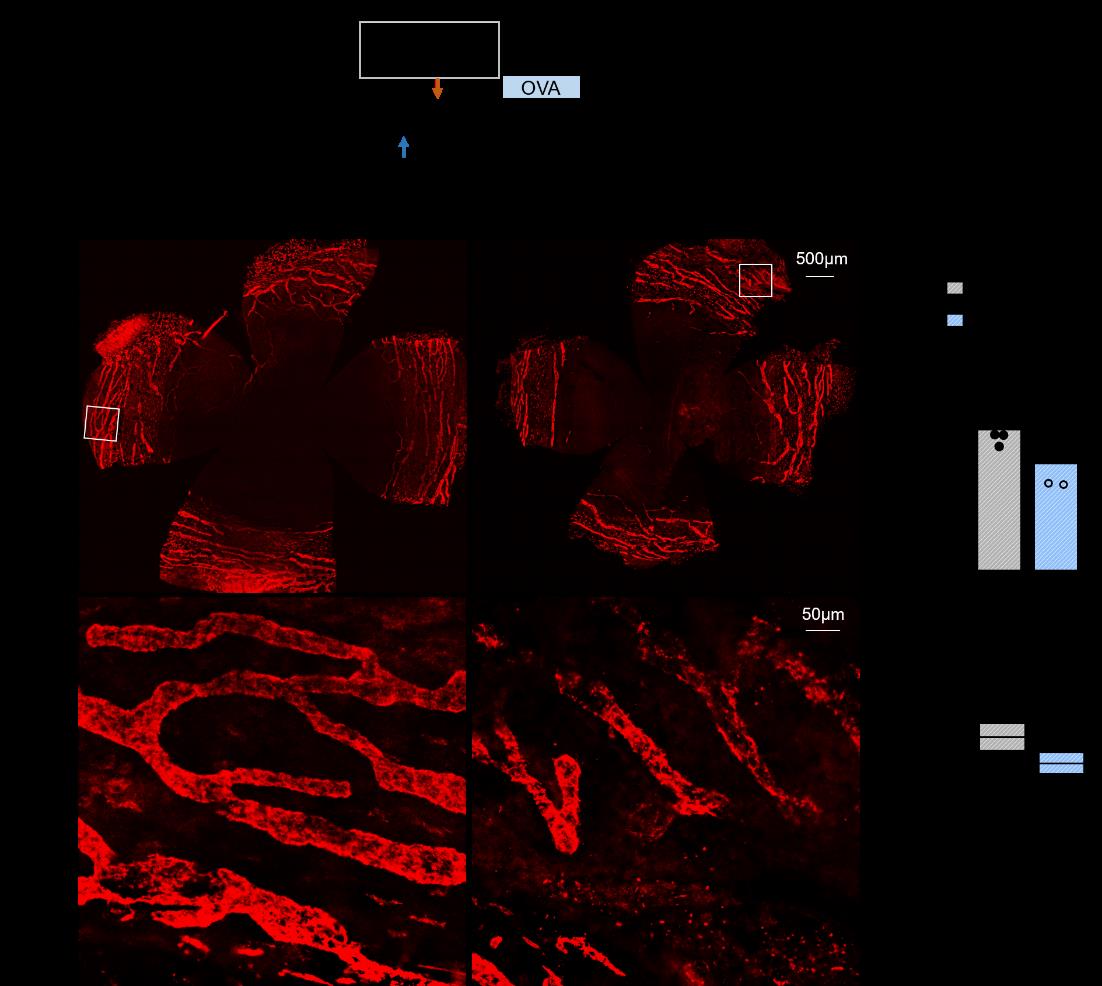
Figure 2 Deletion of lymphatic Vegfr3 prevents allergy-induced conjunctival lymphangiogenesis
To systematically evaluate the role of pro-lymphangiogenic VEGFR3 signal in AED, they performed a differential gene expression analysis on the RNAseq data from unchallenged Ctrl, OVA-challenged Ctrl and OVA-challenged Vegfr3iLECko mouse conjunctiva. With gene set enrichment analysis (GSEA), they determined that OVA challenge led to upregulated gene expression of antigen processing and presentation pathways via both MHC class I and II and of macrophage chemotaxis and differentiation pathways in accordance with the increased density of CD11b+ macrophages in the conjunctival tissue, as indicatives of activation of macrophage-mediated antigen presentation upon allergy. These pathways were significantly inhibited by the deletion of lymphatic Vegfr3, indicating a key role of pro-lymphangiogenic VEGFR3 signal in regulating macrophage-mediated antigen presentation during ocular allergy (Figure 3).
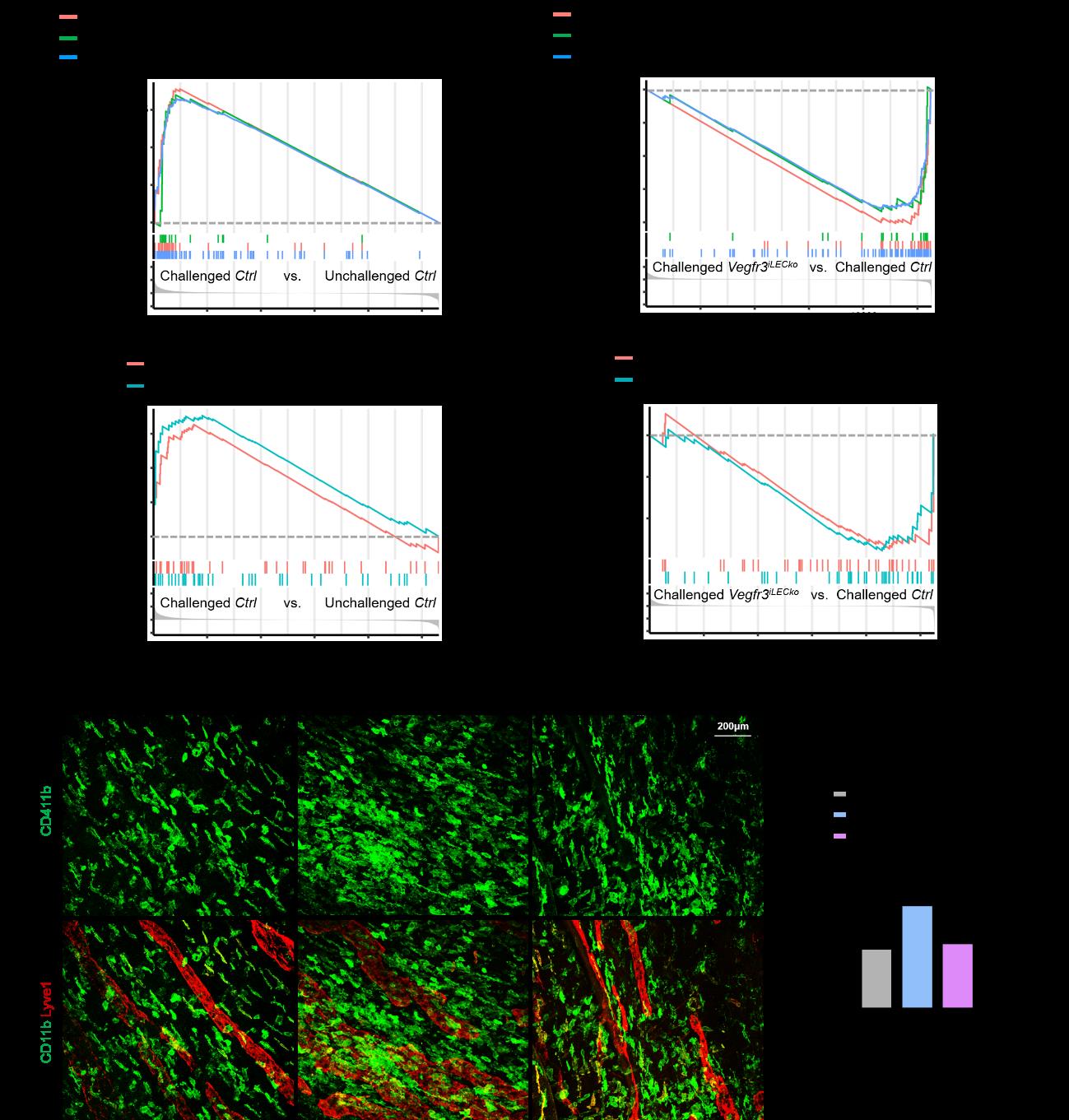
Figure 3 Lymphatic Vegfr3 deletion suppresses antigen presentation pathways in the allergic conjunctiva.
Furthermore, GSEA and histopathological examination also showed that lymphatic VEGFR3 critically contributed the type 2 immune response associated with AED. Lymphatic VEGFR3 signal promoted to the production of the Th2 cell cytokines IL4 and IL10 and the activation/differentiation of CD4+ ab T cells, which are essential pathways of the type 2 immune response (Figure 4).
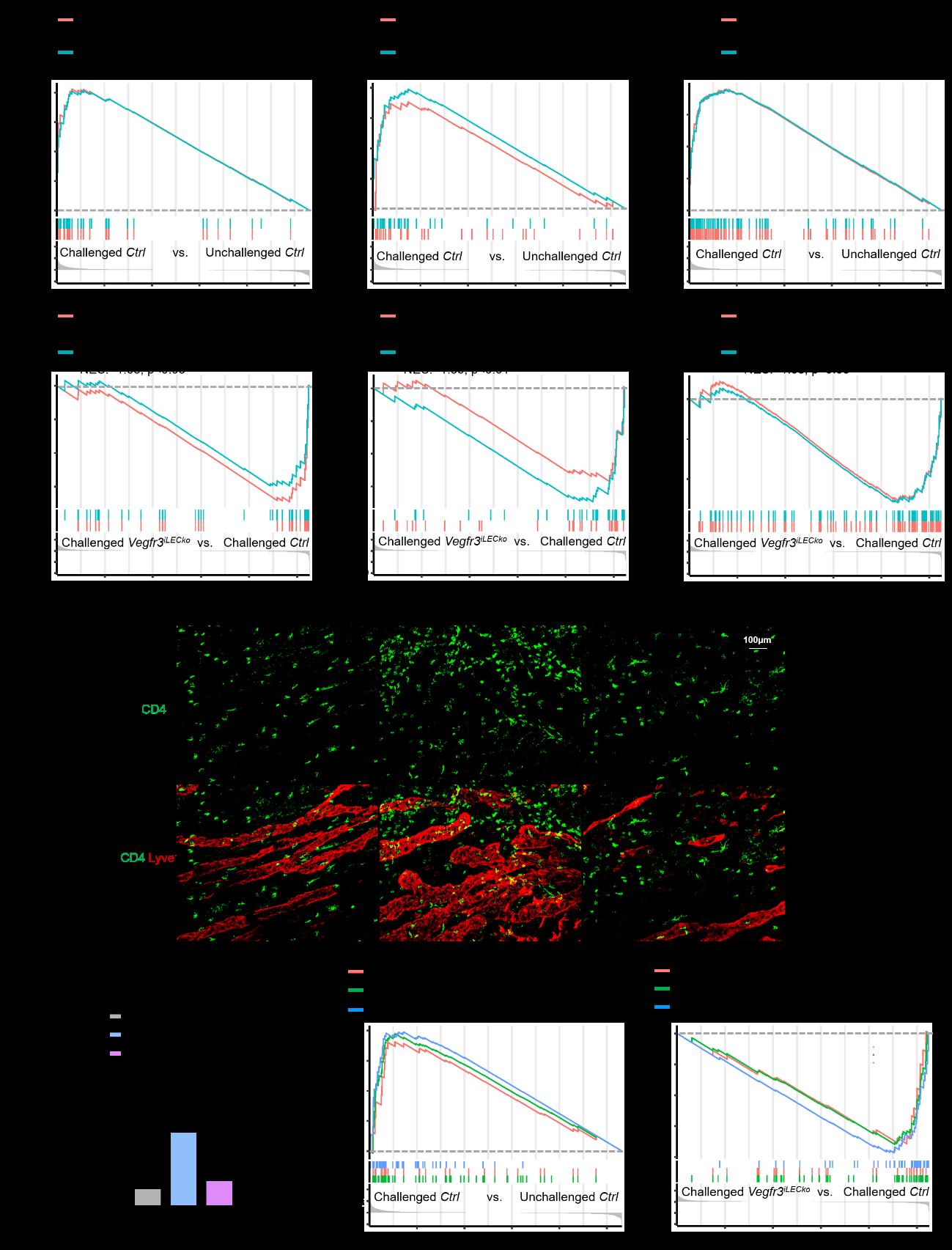
Figure 4 Lymphatic Vegfr3 deletion inhibits Th2 immune response pathways in the allergic conjunctiva
Next, they found lymphatic Vegfr3 significantly inhibited gene expression in B cell activation, immunoglobulin production, mast cell activation pathways in conjunctival tissue after OVA challenge as well as serum levels of total and OVA-specific IgE, suggesting a critical role of pro-lymphangiogenic VEGFR3 signal in promoting B cell-mediated humoral immunity associated with ocular allergy. OVA challenge-induced enlargement of cervical lymph nodes that drain the ocular surface was reversed after lymphatic Vegfr3 deletion and notably, Vegfr3iLECko mouse eyes displayed significantly mitigated clinical manifestations of AED including conjunctival chemosis, hyperemia, discharge/tearing and edema (Figure 5).
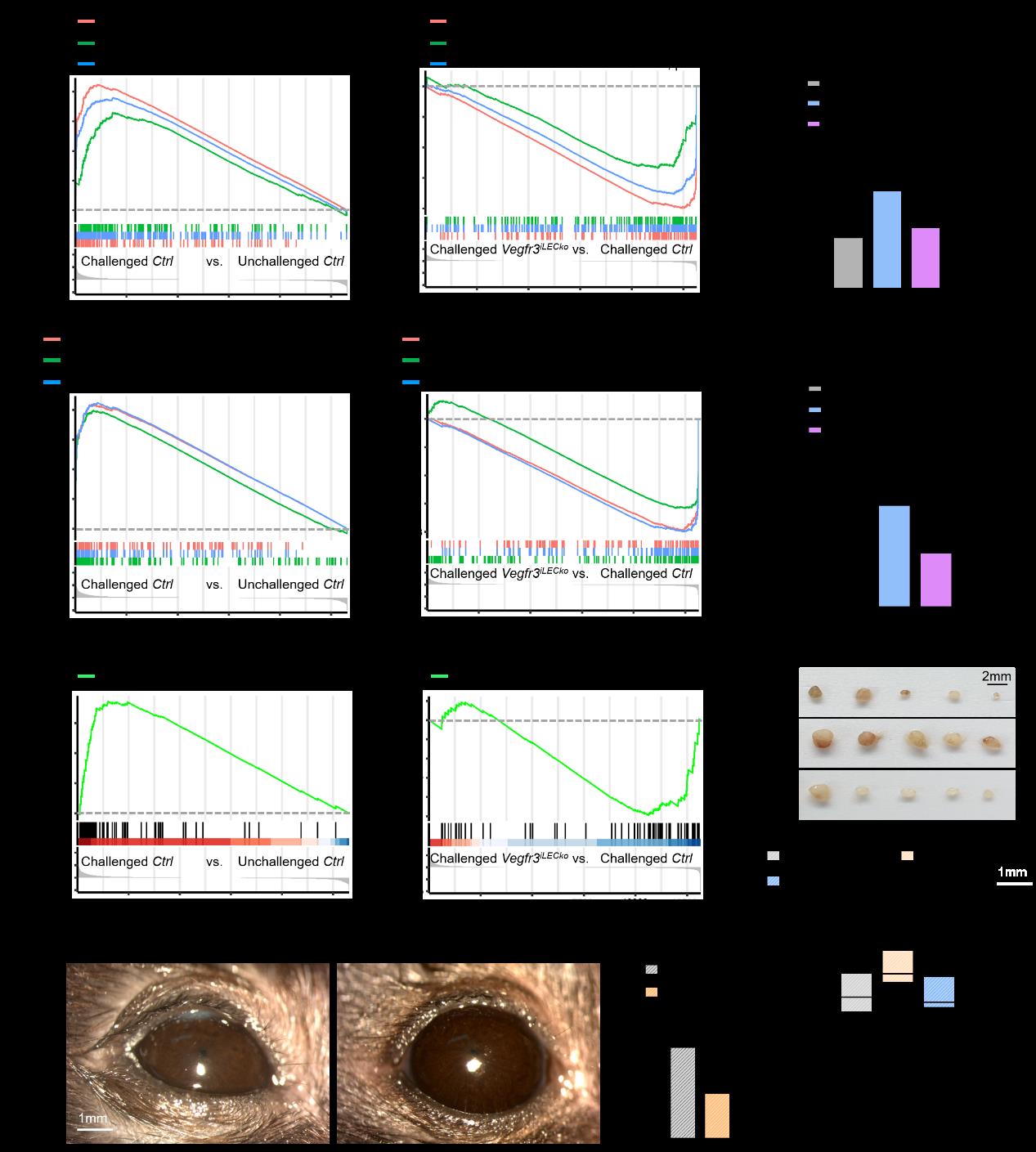
Figure 5 Ablation of lymphatic Vegfr3 inhibits IgE production and alleviates AC manifestations
Lastly, they determined deletion of lymphatic Vegfr3 did not significantly alter the severity of MG plugging upon OVA challenge or the overall expression profiles of Th17 cell and neutrophil-related pathways. Thus, pro-lymphangiogenic VEGFR3 signal did not appear to contribute to AED-associated MG obstruction (Figure 6).
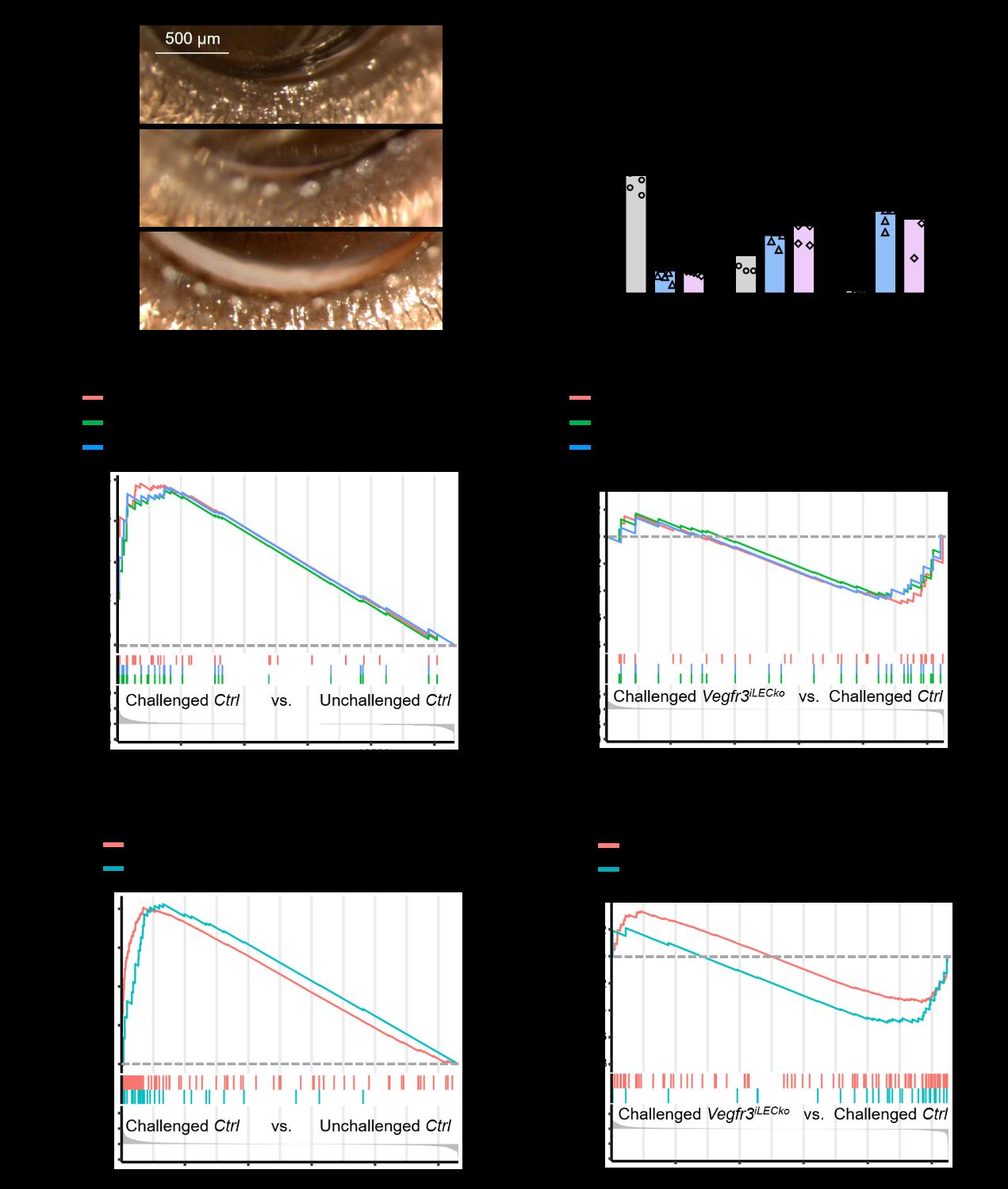
Figure 6 Lymphatic Vegfr3 minimally affects MG obstruction associated with AED
In summary, this work reveals the critical contribution of pro-lymphangiogenic VEGFR3 signal in the development of clinical presentation of AED by coordinating the activation and development of macrophages, Th2 cells, B cells and mast cells that are involved in allergy-induced Th2 immune responses in the conjunctiva, thereby supporting that inhibition of lymphatic VEGFR3 will be beneficial in treating ocular allergic diseases.
